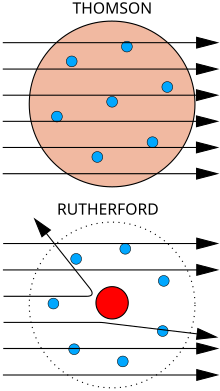
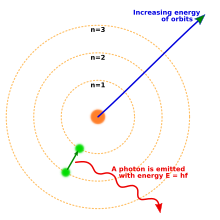
Atomic theory
"Atomic model" redirects here. For the unrelated term in mathematical logic, see Atomic model (mathematical logic).
- This article focuses on the historical models of the atom. For a history of the study of how atoms combine to form molecules, see History of molecular theory.
The word "atom" (from the ancient Greek adjective atomos, 'indivisible'[1]) was applied to the basic particle that constituted a chemical element, because the chemists of the era believed that these were the fundamental particles of matter. However, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called "indivisible atom" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be actually divisible, physicists later invented the term "elementary particles" to describe indivisible particles. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.