Discovery of the nucleus
Main article: Rutherford model
The gold foil experiment
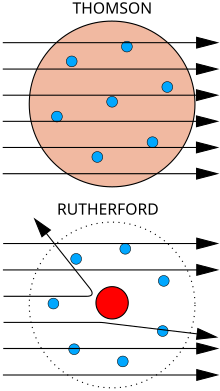
Top: Expected results: alpha particles passing through the plum pudding model of the atom with negligible deflection.
Bottom: Observed results: a small portion of the particles were deflected by the concentrated positive charge of the nucleus.
Top: Expected results: alpha particles passing through the plum pudding model of the atom with negligible deflection.
Bottom: Observed results: a small portion of the particles were deflected by the concentrated positive charge of the nucleus.
In the gold foil experiment, Hans Geiger and Ernest Marsden (colleagues of Rutherford working at his behest) shot alpha particles at a thin sheet of gold, measuring their deflection with a fluorescent screen.[11] Given the very small mass of the electrons, the high momentum of the alpha particles and the unconcentrated distribution of positive charge of the plum pudding model, the experimenters expected all the alpha particles to pass through the gold sheet without significant deflection. To their astonishment, a small fraction of the alpha particles experienced heavy deflection.
This led Rutherford to propose a planetary model in which a cloud of electrons surrounded a small, compact nucleus of positive charge. Only such a concentration of charge could produce the electric field strong enough to cause the heavy deflection.[12]
No comments:
Post a Comment