First steps toward a quantum physical model of the atom
Main article: Bohr model
The planetary model of the atom had two significant shortcomings. The first is that, unlike planets orbiting a sun, electrons are charged particles. An accelerating electric charge is known to emit electromagnetic waves according to the Larmor formula in classical electromagnetism; an orbiting charge should steadily lose energy and spiral toward the nucleus, colliding with it in a small fraction of a second. The second problem was that the planetary model could not explain the highly peaked emission and absorption spectra of atoms that were observed.
The Bohr model of the atom
Quantum theory revolutionized physics at the beginning of the 20th century, when Max Planck and Albert Einstein postulated that light energy is emitted or absorbed in discrete amounts known as quanta (singular,
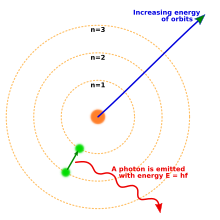
quantum). In 1913, Niels Bohr incorporated this idea into his Bohr model of the atom, in which an electron could only orbit the nucleus in particular circular orbits with fixed angular momentum and energy, its distance from the nucleus (i.e., their radii) being proportional to its energy.
[13] Under this model an electron could not spiral into the nucleus because it could not lose energy in a continuous manner; instead, it could only make instantaneous "quantum leaps" between the fixed energy levels.
[13] When this occurred, light was emitted or absorbed at a frequency proportional to the change in energy (hence the absorption and emission of light in discrete spectra).
[13]
No comments:
Post a Comment